orange book pharmacy definition
National Association of Boards of Pharmacy. One prescription example would be combined oral contraception also.
The Orange Book Introduction.
. Rucha Pathak Roll No. The IUPAC Compendium of Analytical Nomenclature informally known as the Orange Book. Approved Drug Products with Therapeutic Equivalence Evaluations is one option -- get in to view more The Webs largest and most authoritative acronyms and abbreviations resource.
Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for. CDR Kendra Stewart RPh PharmD.
Looking for the definition of ORANGE BOOK. Get emails about this page. Office of Generic Drugs Policy Center for Drug Evaluation Research US.
The orange book is a list of generic drugs approved by FDA. Reclaiming Liberalism by members of the British Liberal Democrat party. See note 5 supra.
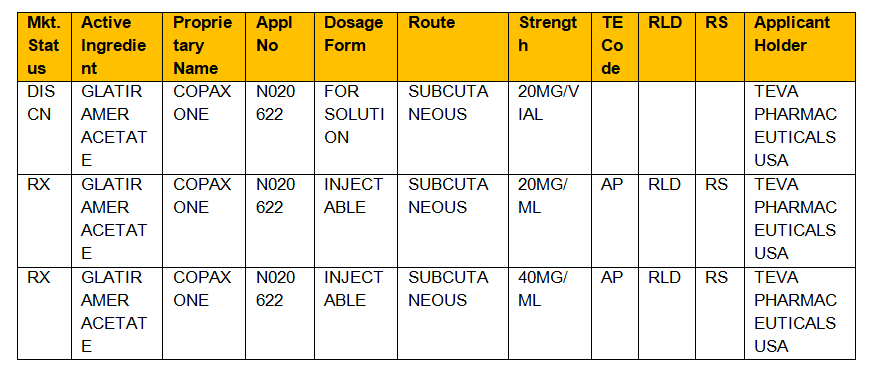
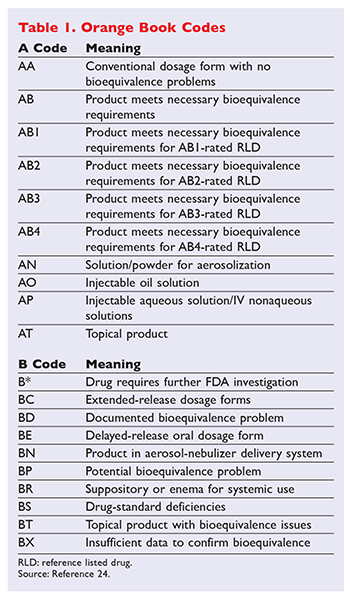
In the electronic Orange Book a reference standard is identified by RS in the RS column. Approved Drug Products with Therapeutic Equivalence Evaluations. Approved Drug Products with Therapeutic Equivalence Evaluations published by the FDAs Center for Drug Evaluation and Research.
Originally this book was published in October 1980 with orange cover and thus the name orange book. Sumanta Mondal_MPhar m 1 th Sem. The orange book consist of five main sections.
FDAs Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book identifies drug products approved on the basis of safety and effectiveness. Drug product selection laws. The FDA keeps a list known as the Orange Book of every approved therapeutic equivalent.
FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations. _ GITAM Institute of Pharmacy. Tap card to see definition.
Survey of Pharmacy Laws. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Therapeutic equivalence evaluations in this publication are not official FDA actions affecting the legal status of products under the Act See note 5 supra.
The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through. Updated with Orange Book. Back to top I want drug information not found in the Orange Book.
The Orange book has been revised. G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication. Click card to see definition.
It is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products. Before understanding different drug ratings it is necessary. Electronic Orange Book.
The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDA. R i s k r e. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use.
Format is RX OTC DISCN. First published in 1971 the original Orange Guide contained British Good Manufacturing Practice and was entitled Guide to Good Pharmaceutical Manufacturing Practice. Drug pricing data AWP Found in Micromedex Truven Published by Drug Topics hard copy.
The Orange Book Risk Management Principles. Generic Drugs and The Orange Book. Basics in drug approval process with reference to the Orange Book Presented by.
Type The group or category of approved drugs. In the Orange Book there are links on column headings that will link to the definition of the term. Not much more than 30 pages in length this voluntary guide was an aid to manufacturers to understand the needs of the regulatory authoritys requirements for the manufacture of.
Food and Drug Administration. See note 7 supra. The orange book is available in electronic format Electronic Orange Book to provide access to information such as.
It is the publication of.
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

Otc Introduction And Definitions Ppt Video Online Download

The Introduction Of An Orange Book

The Introduction Of An Orange Book

Investigational New Drug Orange Book Understanding On 505 B 2 A

Orange Book And Its Applications Legal Advantage

Investigational New Drug Orange Book Understanding On 505 B 2 A

A New Social History Of Pharmacy Pharmaceuticals Festival American Institute Of The History Of Pharmacy

The Introduction Of An Orange Book
Therapeutic Equivalence Definition Examples Video Lesson Transcript Study Com

The Introduction Of An Orange Book

The Introduction Of An Orange Book
Insights Into Effective Generic Substitution

Investigational New Drug Orange Book Understanding On 505 B 2 A
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)